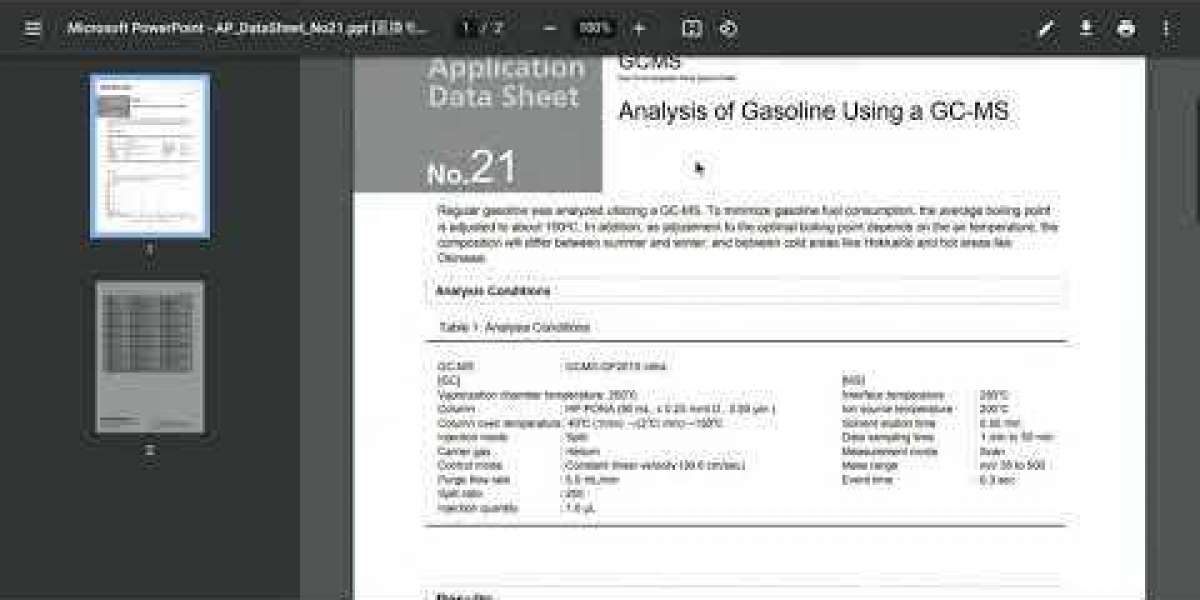
Chromatography can be carried out in a variety of ways, the most common of which are high-performance liquid chromatography (HPLC) and gas chromatography machine (GC), amongst others.
The components of a mixture can be identified using this method by timing how long it takes a sample to travel through a stationary phase and calculating the time difference between the two.
The abbreviation for gas chromatography machine is GC.
Gas chromatography, also known as gas-liquid chromatography, is another common type of chromatography method. Gas chromatography was first developed in the early 1900s. Using this method, volatile samples, which are typically gases when they are at room temperature, are analyzed. In order to finish the analysis, a gaseous mobile phase is used to transport the sample through a solid stationary phase. This is done using a chromatograph.
When it comes to the separation of the compounds in the sample that takes place in the column, the length of the column, the temperature of the column, and the flow rate of the carrier gas are all important factors to consider.
Comparisons between gas chromatography and liquid chromatography and their key differences
Differences in Approaches and Methods
The techniques of gc instrument (GC) and high-performance liquid chromatography (HPLC) are fundamentally distinct from one another in a number of significant respects, including the following:
Using the Mobile Phase as our Base
Both high-performance liquid chromatography (HPLC) and gas chromatography machine (GC) derive their names from the mobile phase, which is also the primary difference between the two techniques. HPLC is used to analyze substances using liquids, while GC analyzes substances using gases.
In contrast, gas chromatography, also known as GC, employs the utilization of a gas that is either non-reactive or inert, and this gas is also referred to as the carrier gas. The type of gas that is utilized will be decided by the method of detection that is applied at the very end of the process. In most cases, the cause of this issue is determined to be the polarity of the sample in relation to the phases.
The more polar components will move through the column at a quicker rate due to the fact that they are drawn to the mobile phase to a greater extent.
In contrast, separation in GC happens according to the relative volatility of each component of the sample that is being analyzed. This is a key difference between the two methods. High-performance liquid chromatography, also known as HPLC, is the analytical technique of choice for analyzing soluble compounds, which may or may not be volatile.
However, in order to use GC, the sample mixture needs to be volatile. This normally denotes that the substance in question exists in the form of a gas when it is allowed to reach room temperature. This demonstrates that GC is typically used as a method of separation for air samples and a variety of other organic compounds that cannot be identified. In point of fact, the temperature of the column in gc instrument is typically between 150 and 300 degrees Celsius, whereas the operating temperature in liquid chromatography is room temperature, which is approximately 20 to 25 degrees Celsius. Liquid chromatography is performed at a lower temperature than gas chromatography.
The process of can become somewhat more difficult as a consequence of this, and increased caution is required when manipulating equipment such as the columns.
Elution via GC can be completed in as little as a few seconds or as much as a few minutes, depending on how long the gradient is. The speed of elution is ultimately determined by the sample that is being tested in conjunction with the flow rate of the carrier gas. On the other hand, it may be difficult to differentiate between compounds if they share similar properties, which would cause their retention times to be indistinguishable from one another and therefore make it more difficult to differentiate between them.
A gas chromatogram may have a low resolution for substances that have molecular weights that are comparable to one another because GC is dependent on volatility. In a manner not dissimilar to the previous example, the polarity of compounds can be a factor in the lack of resolution in liquid chromatograms. A column that has an internal diameter of can be as long as one hundred meters in some procedures, and gas chromatography machine can be as short as one meter in others.
Made Use Of A Solvent
Polar characteristics are required of the solvent being used in high-performance HPLC; otherwise, the desired results cannot be obtained. In HPLC, the use of solvents is typically comprised of both water and methanol.
Identification
The techniques of detection employed by liquid chromatography and are distinct from one another, and the differences between the two can be quite substantial.
The non-destructive high-performance liquid chromatography (HPLC) detection method typically makes use of both an ultraviolet-visible (UV/Vis) spectroscopic detector as well as a refractive index detector (RID). For the (GC) analysis, another choice would be to make use of a thermal conductivity detector (TCD), which is a method of detection that can be applied to any substance.
In addition, the utilization of gas generators can, over the course of time, bring the cost of GC down to an even more manageable level. These generators ensure that the carrier gas is available whenever it is required, which eliminates the need for expensive storage and delivery services.
Oils, organic compounds, air samples, toxins, and drugs (both pharmaceutical and recreational) are the typical kinds of substances that are measured by GC. GC can also be used to analyze drug levels in recreational drug use.
The most common uses for high-performance liquid chromatography (HPLC) involve the separation of inorganic ions, food substances like sugars, proteins, and vitamins, and other compounds like polymers, nucleotides, and tetracyclines.
When carrying out analyses, it is of the utmost importance to guarantee that the apparatus being used is in proper working order in order to produce accurate results. This is just as significant as choosing the right apparatus to use. The use of faulty equipment, such as columns that have been damaged or detectors that have been compromised, can lead to inaccurate results and the incorrect identification of molecules. Examples of this type of equipment include damaged columns and compromised detectors. We also offer comprehensive maintenance contracts that include expert help in the event that you run into problems with the laboratory equipment you have purchased from us.